16+ Alkane To Alkene Conditions
Alkane To Alkene Conditions. Here, we demonstrate the hydrogenolysis of polyethylene into liquid alkanes under mild conditions using ruthenium nanoparticles supported on carbon (ru/c). Alkanes, which are major constituents of crude oils, can be degraded under extreme conditions, both aerobically and anaerobically by bacteria and archaea of different phyla.

Halogen bonds and broken producing reactive intermediates called free radicals. Alkanes, which are major constituents of crude oils, can be degraded under extreme conditions, both aerobically and anaerobically by bacteria and archaea of different phyla. Alkenes, purple kmno 4 is decolourised.
artens camden naturel old 2006 chrysler 300 hemi specs 30mm round accessoire robot bosch mum54a00
Difference Between Ethane and Ethanol Compare the
A type of structural isomerism in which the functional group is in a. Brown ppt of mno 2 is formed in alkaline condition. Therefore, to convert an alkene to an alkyne, you simply need to break the double bond. Halogen bonds and broken producing reactive intermediates called free radicals.

An alkane, on the other hand, is a hydrocarbon with only single bonds. To this end, we report a highly enantioselective, intermolecular hydroamination of structurally diverse unactivated terminal alkenes under mild conditions. Reactions of alkenes product type of reaction (name) reaction conditions regiochemistry stereochemistry halides (ch 6.9) electrophilic addition hx, organic solvent (anhydrous) markovnikov addition no stereochemical pref. The only.
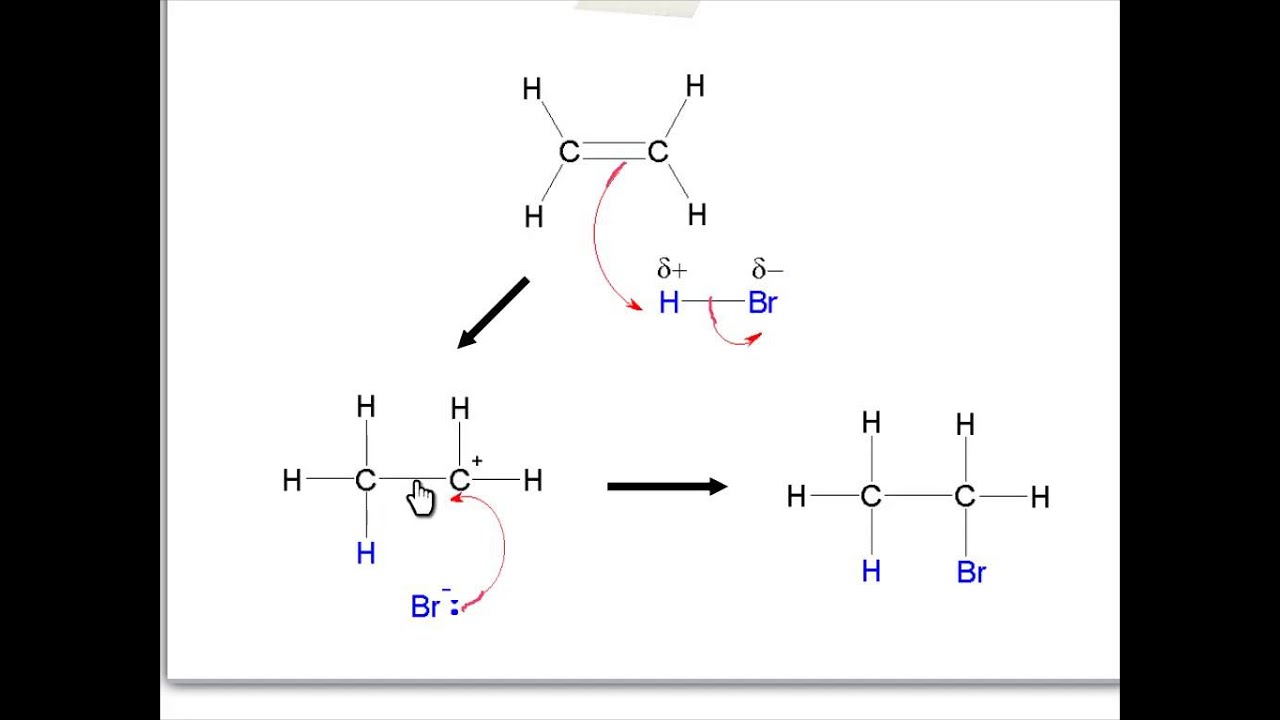
Reactions of alkenes product type of reaction (name) reaction conditions regiochemistry stereochemistry halides (ch 6.9) electrophilic addition hx, organic solvent (anhydrous) markovnikov addition no stereochemical pref. Alkenes, purple kmno 4 is decolourised. To do so, you can use the. Brown ppt of mno 2 is formed in alkaline condition. Alkanes and alkenes are both families of hydrocarbons.

I haven't included the mechanism for the hydration of these more complicated alkenes anywhere on the site, but it isn't too difficult to work out for yourself if you know the mechanism for the hydration of ethene , and know about the stability of. What is initiation in free. Complete combustion needs plenty of air. Three denitrifying isolates (strains hxn1,.
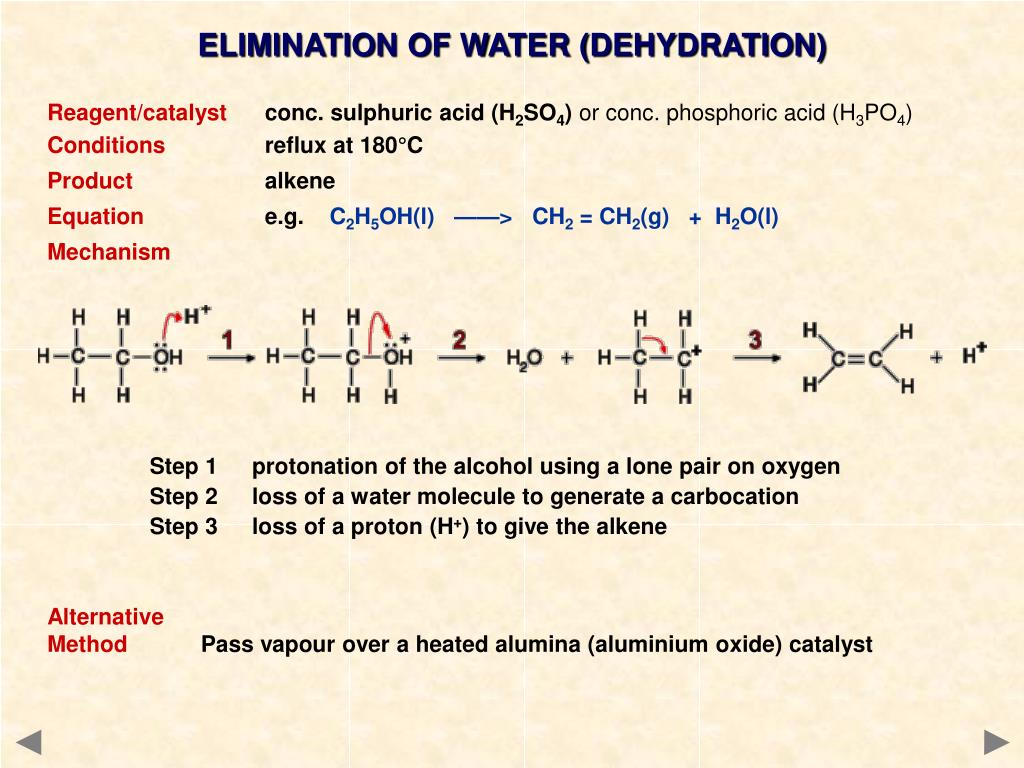
A precatalyst crucial for this reaction was. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds. Alkenes can react with different types of chemicals during addition reactions. The colour change depends on whether the potassium manganate(vii) is used under acidic or alkaline conditions. • the reaction uses h2 and a precious.